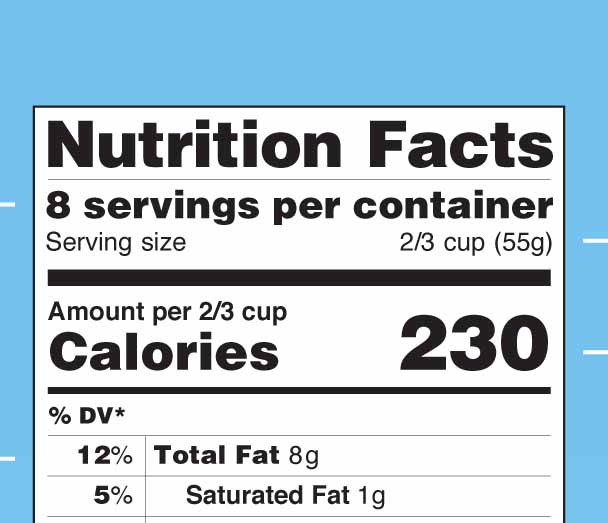
Americans may soon see more realistic serving sizes and more prominent calorie counts on the labels of their favorite soft drink or cereal box.
The Food and Drug Administration proposed several changes to the Nutrition Facts label on Feb. 27. University of Georgia nutrition experts and researchers believe the proposed changes, which have not been finalized, will help Georgians make more informed food choices.
The changes include making calorie counts and serving sizes more prominent on labels, making serving sizes more realistic, requiring the amount of added sugar, vitamin D and potassium in products and adjusting the recommended daily amounts of fiber, sodium and vitamin D. The FDA’s recommended amounts of fiber and sodium haven’t been changed in more than 40 years.
All of the the proposed changes reflect the latest scientific findings, such as the link between diet and chronic diseases like obesity and heart disease, according to the FDA.
“Overall, I am very pleased with the proposed changes,” said Connie Crawley, a UGA Extension nutrition and health expert. “I basically approve of all of them, especially updating the percent daily values that are based on 1968 recommended dietary allowances and listing added sugars.”
Another change would remove the “calories from fat” item on current labels. According to the FDA release, research shows the type of fat is more important than the amount.
“Leaving off calories from fat is probably a good idea,” Crawley said. “It was poorly understood, and now we are more interested in the quality of fat and not so much the quantity of fat.”
The nutritional facts label has been required on food packages for 20 years. It hasn’t changed significantly since 2006 when transfat information was included, according to the FDA.
“This is a huge positive for consumers,” said Kelly Pritchett, assistant professor for sports nutrition within UGA’s Department of Foods and Nutrition. “These modifications are a direct reflection of the Academy of Nutrition and Dietetics evidence-based practice recommendations for healthful eating.”
While both Crawley and Pritchett stressed the public will benefit from additional education about the information found on food labels, such as the difference between “natural sugars” and “added sugars,” they agreed the proposed changes are a positive step.
The agency is accepting public comment on the proposed changes for 90 days. A full listing of the proposed changes and explanations behind each proposed change are available at www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm387418.htm.